
Polar and Nonpolar Covalent Bonds Characteristics & Differences
It is a reddish-brown liquid that has a pungent odor and is highly toxic. BrCl3 is a powerful oxidizing agent and reacts violently with water, making it a hazardous substance to handle. What Makes a Compound Polar or Nonpolar? Before we determine whether BrCl3 is polar or nonpolar, we need to understand what makes a compound polar or nonpolar.
Is bcl3 polar or nonpolar
a) a change in mass of an electron emitted from the nucleus b) decreasing mass by one unit c) increasing mass by one unit d) adding a proton to the nucleus e) converting 1 1 neutron to 1 1 electron and 1 1 proton Science Chemistry Question Is BrCl polar or nonpolar? Solution Verified Answered 1 year ago Create a free account to view solutions
Bcl3 é Polar Ou Apolar EDUCA
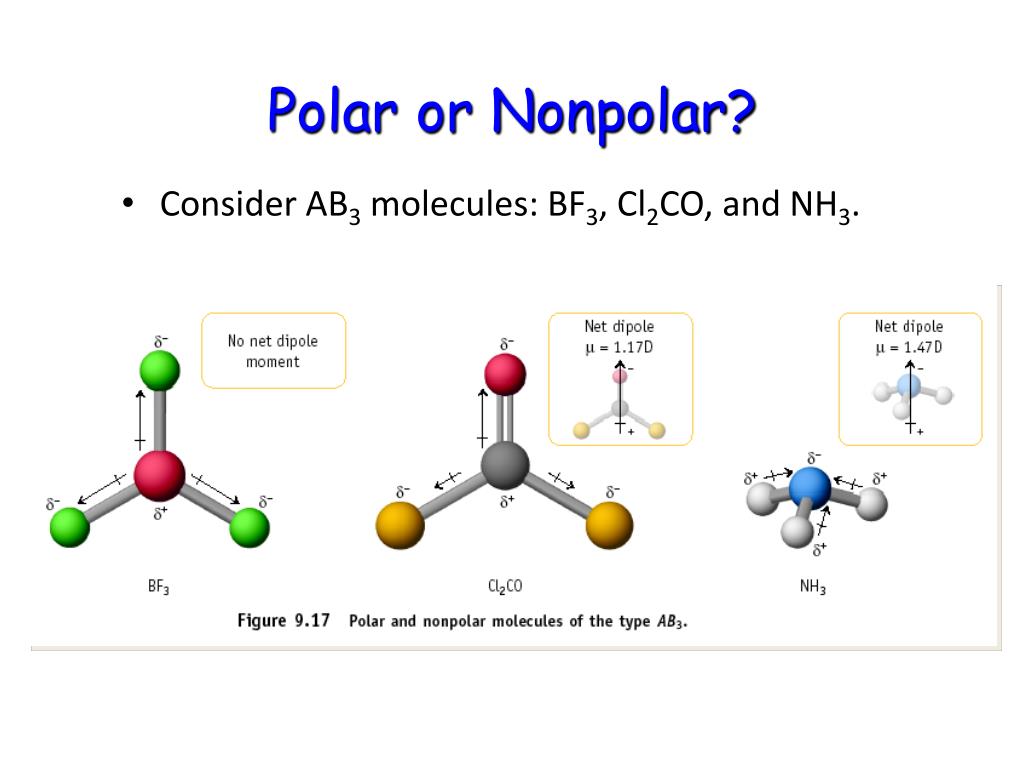
Specify the polarity (polar or nonpolar) for each of the four molecules. arrow_forward Molecules in space: (a) In addition to molecules such as CO, HCl, H2O, and NH3, glycolaldehyde has been detected in outer space.

Brcl3 lewis structure 👉👌Drawing Lewis structures ppt download
Before we dive into whether BrCl3 is polar or nonpolar, it is essential to understand what polarity is. The polarity of a molecule is determined by the electronegativity of its atoms. Electronegativity is the measure of an atom's ability to attract electrons towards itself.

Reading Covalent Bonds Biology I
Chemistry Chemistry questions and answers On your Calcs page, draw a Lewis structure for BrCl3 and answer the following questions: a. What is the electron group geometry of BrCl3? b. What is the molecular geometry of BrCl3 ? c. What is the hybridization of the central atom in BrCl3? Here in the test, choose whether BrClz is POLAR or NONPOLAR.

Is BrCl3 Polar or Nonpolar (Bromine Trichloride) YouTube
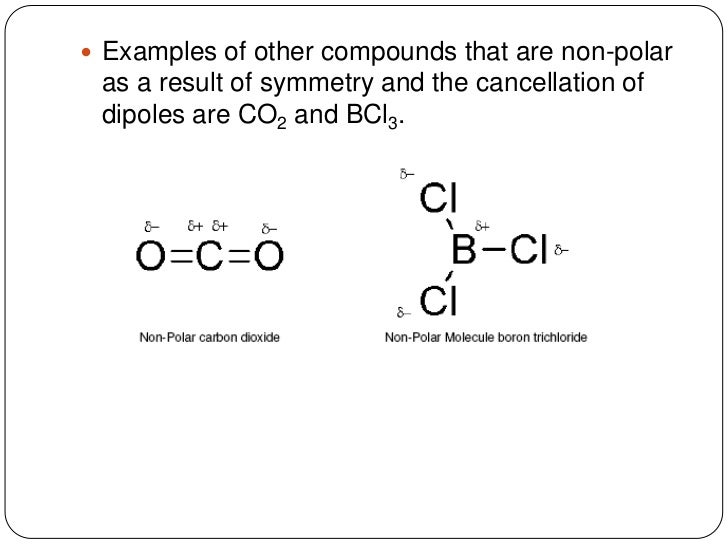
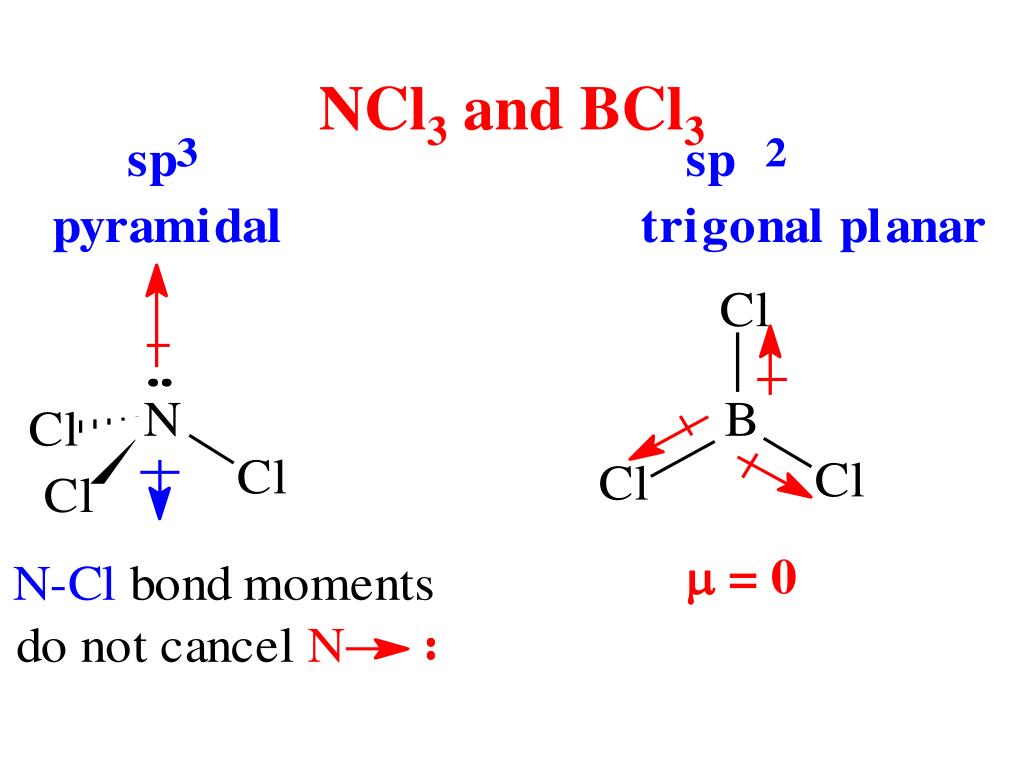
Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie; Trigonal Planar. The B-Cl bond itself is polar because of the difference in electronegativity of Boron (2.04) and Chlorine (3.16) atoms and all three B-Cl bonds lie at 120 degrees to each other.
Is BCl3 Polar or Nonpolar? (Boron Trichloride) YouTube
Bromine monochloride is an inorganic compound composed of Br and Cl atoms. Both of these are halogens, and BrCl belongs to a class of interhalogen compounds. BrCl is a golden yellow gas at room temperature. It is very reactive and unstable. Bromine monochloride's boiling and melting points are 5°C and -66°C, respectively. It has an irritating odor.

PPT Molecular Geometry and Polarization PowerPoint Presentation, free
in Science 0 BRCL3 | Bond Angle, Molecular Geometry & Hybridization | Polar or Non Polar Bromine Trichloride Bromine trichloride (BrCl3) is an organic chemical composed of one bromine atom and three chlorine atoms. It's a brownish-red liquid with a strong odor at room temperature and pressure.

(Get Answer) ) 8) Bromine trichloride BrCl3 Lewis structure Electron
Successive substitution of F atoms for H atoms in the molecule NH3 produces the molecules NH2F, NHF2, and NF3. a. Draw Lewis structures for each of the four molecules. b. Using VSEPR theory, predict the geometry of each of the four molecules. c. Specify the polarity (polar or nonpolar) for each of the four molecules.

Polar and Nonpolar Molecules Covalent bonding, Chemistry lessons
Chemistry Chemistry questions and answers Draw the Lewis structure for BrCl3 in the Marvin window below and then answer the questions that follow. (a) What is the electron-pair geometry for Br in BrCl3? (b) What is the the shape (molecular geometry) of BrCl3? This problem has been solved!
Bcl3 Polar Or Nonpolar Covalent Bond Polar vs. Nonpolar Bonds
FLAME RETARDANT. Hazardous Substances Data Bank (HSDB) Addition /to isoprene / is induced using bromotrichloromethane in a 3:1 mol ratio to isoprene by x-irradiation at 60 Gy/min. The main reaction yields a mixture of ca 75% 1,4 and 25% 4,1 addition product. Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY.

Bcl3 é Polar Ou Apolar EDUCA
Boron trichloride, or BCl3, is nonpolar. The three chloride atoms have a negative charge, and the one boron in the center has an equal but positive charge. Boron sits in the center of the molecule and has three valence electrons, so it balances out the three chlorides.

Is \ce{BrCl3} polar molecule? Quizlet
Brcl3 exists as a reddish-brown liquid at room temperature, emitting a pungent odor. It is insoluble in water but can dissolve in organic solvents such as chloroform and carbon disulfide. The boiling point of brcl3 is around 102 degrees celsius. Brcl3 is highly reactive and acts as both an oxidizing agent and a halogenating agent.
What Is The Difference Between Lewis Structure And Vsepr
Learn to determine if BCl3 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then us.
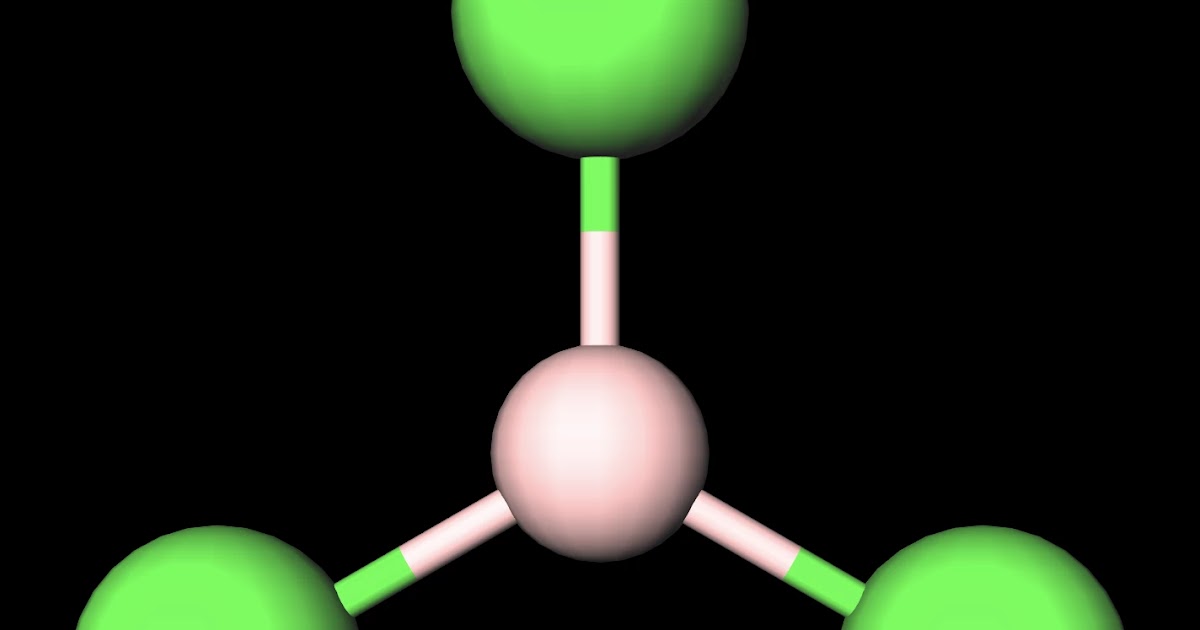
MakeTheBrainHappy Is BCl3 Polar or Nonpolar?
1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 Share 1.2K views 1 year ago Polarity of Molecules Hello Everyone, welcome back to Geometry of Molecules, where we make Chemistry fun and easy. For today's.

BrCl Lewis Structure, Geometry, Hybridization, and Polarity
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.